![]() Yamaguchi University School of Medicine
Yamaguchi University School of Medicine
Department of Biochemistry and Molecular Biology
Research Activities
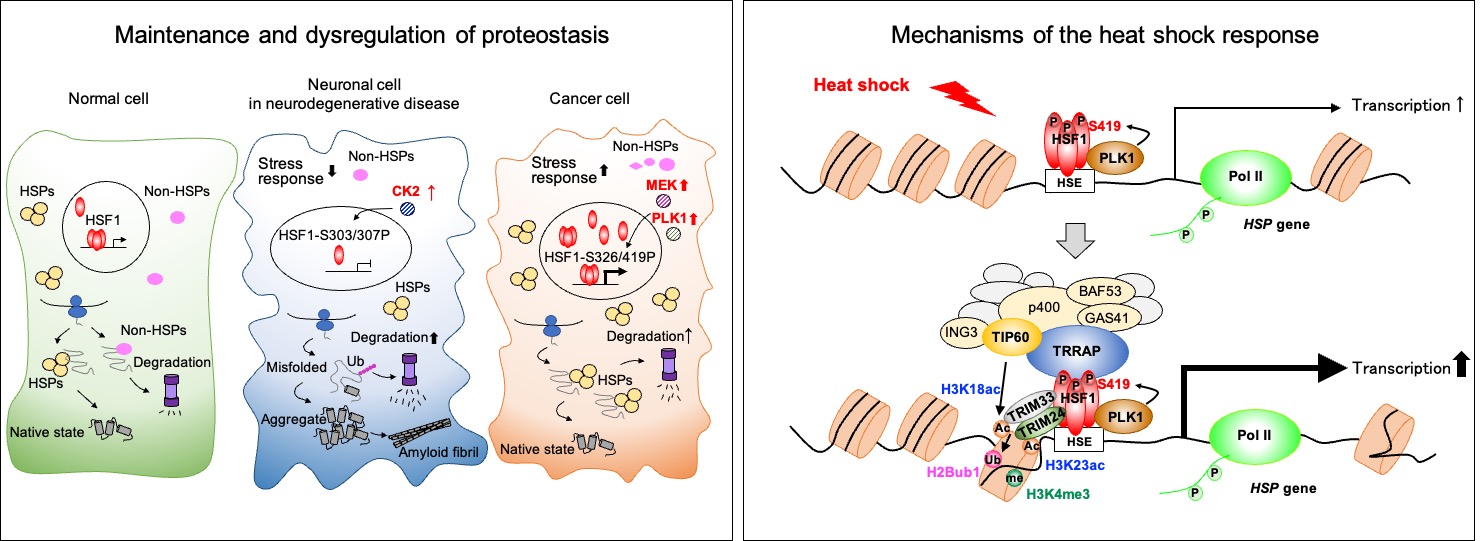
Aims of our researches are to understand molecular mechanisms of regulation of proteostasis capacity by focusing on the heat shock response and heat shock factors (HSFs), and to find new strategies for ameliorate protein misfolding diseases and cancers.
Proteostasis capacity is reduced during ageing, and is associated with age-related protein misfolding deaseses such as neurodegenerative diseases. HSF1 and other HSF family members play a pivotal role in regulating proteostasis capacity within a cell. Therefore, a gain of HSF1 function inhibits progression of protein misfolding deasese in mice and extends lifespan in C. elegans. Conversely, a loss of HSF1 function suppresses cancer progression, because initiation and progression of cancer are dependent on an elevated proteostasis capacity via HSF1 activation.
Recently, we are analyzing components of HSF1 transcriptional complex and uncovering signaling pathways, which connect cellular metabolisms to proteostasis capacity. We already show that the proteostasis capacity is tightly linked with DNA, glucose, lipid, and energy homeostasis in a cell. These analyses should identify novel therapeutic targets for the treatment of patients suffered from protein misfolding deaseses and cancers.
Selected Publications
1) Takii R, Fujimoto M, Pandey A, Jaiswal K, Shearwin-Whyatt L, Grutzner F, and Nakai A. HSF1 is required for cellular adaptation to daily temperature fluctuations. Sci. Rep. 14, 21361, 2024, September 12, 2024, DOI: 10.1038/s41598-024-72415-x.
2) Okada M, Fujimoto M, Srivastava P, Pandey A, Takii R, and Nakai A. The Mediator subunit MED12 promotes formation of HSF1 condensates on heat shock response element arrays in heat-shock clles.FEBS Lett. 597 (13), 1702-1717, 2023 (selected as the Editor's Choice)
3) Fujimoto M, Takii R, Matsumoto M, Okada M, Nakayama KI, Nakato R, Fujiki K, Shirahige K, and Nakai A. HSF1 phosphorylation establishes an active chromatin state via the TRRAP-TIP60 complex and promotes tumorigenesis. Nat. Commun. 13, 4355, 2022. DOI: 10.1038/s41467-022-32034-4. (featured in a Nature Communications Editors’ Highlights webpage) (Press release)
4) P. Srivastava, R. Takii, M. Okada, M. Fujimoto, and A. Nakai. MED12 interacts with the heat shock transcription factor HSF1 and recruits CDK8 to promote the heat shock response in mammalian cells. FEBS Lett. 595 (14), 1933-1948, 2021. DOI: 10.1002/1873-3468.14139.
5) A. Katiyar, M. Fujimoto, K. Tan, A. Kurashima, P. Srivastava, M. Okada, R. Takii, and A. Nakai. HSF1 is required for induction of mitochondrial chaperones during the mitochondrial unfolded protein response. FEBS Open Bio 10 (6), 1135-1148, 2020. (selected as the winner of inaugural 2021 FEBS Open Bio Prize)
6) R. Takii, M. Fujimoto, M. Matsumoto, P. Srivastava, A. Katiyar, K.I. Nakayama, and A. Nakai. The pericentromeric protein shugoshin 2 cooperates with HSF1 in heat shock response and RNA Pol II recruitment. EMBO J. 38 (24), e102566, 2019. (selected as a Cover, Introduced in News & Views) (Press release)
7) M. Fujimoto, R. Takii, A. Katiyar, P. Srivastava, and A. Nakai. Poly(ADP-Ribose) Polymerase 1 Promotes the Human Heat Shock Response by Facilitating Heat Shock Transcription Factor 1 Binding to DNA. Mol. Cell. Biol. 38, e00051-18, 2018.
8) M. Fujimoto, R. Takii, E. Takaki, A. Katiyar, R. Nakato, K. Shirahige, and A. Nakai. The HSF1-PARP13-PARP1 complex facilitates DNA repair and promotes mammary tumorigenesis. Nat. Commun. 8, 1638, 2017. (Press release)
9) K. Tan, M. Fujimoto, R. Takii, E. Takaki, N. Hayashida, and A. Nakai. Mitochondrial SSBP1 protects cells from proteotoxic stresses by potentiating stress-induced HSF1 transcriptional activity. Nat. Commun. 6, 6580, 2015. (Press release)
10) R. Takii, M. Fujimoto, K. Tan, E. Takaki, N. Hayashida, R. Nakato, K. Shirahige, and A. Nakai. ATF1 modulates the heat shock response by regulating the stress-inducible HSF1-transcription complex. Mol. Cell. Biol. 35, 11-25, 2015.
11) M. Fujimoto, E. Takaki, R. Takii, K. Tan, R. Prakasam, N. Hayashida, S. Iemura, T. Natsume, and A. Nakai. RPA assists HSF1 access to nucleosomal DNA by recruiting histone chaperone FACT. Mol. Cell 48, 182-194, 2012. (Press release)
Selected Reviews
1) Fujimoto M, Takii R, and Nakai A. Regulation of HSF1 transcriptional complexes under proteotoxic stress. BioEssays 45 (7), e2300036, 2023. (Invited Review)
2)Nakai A. Molecular basis of HSF regulation. Nat. Struct. Mol. Biol. 23, 93-95, 2016 (News and Views)
3) Nakai A ed. 2016. Heat Shock Factor. Springer, Japan. (eBook + Hard Cover)
4) Fujimoto M, Takii R, Hayashida N, Nakai A. Analysis of the heat shock factor complex in mammalian HSP70 promoter. Methods Mol. Biol. 1292, 53-65, 2015.
5) A. Nakai. Novel aspects of heat shock factors. FEBS J. 277: 4111, 2010. (Overview of the Mini-review series)
6) M. Fujimoto and A. Nakai. Novel aspects of heat shock factors: the heat shock factor family and adaptation to proteotoxic stress. FEBS J. 277: 4112-4125, 2010. (FEBS Journal Top-Cited Paper Award)
7) A. Nakai. Heat shock transcription factors and sensory placode development. BMB reports 42: 631-635, 2009.
Message
A cell is a fundamental unit of life. Researchers in Biochemical and Molecular Biological fields are trying to understand elementary processes in cells that regulate cell growth, differentiation, death, and responses to stimuli. Discovery of a new elementary process contribute not only on establishing a novel concept but also on understanding and treatment of diseases. Our laboratory welcomes researchers who are enthusiastic to find out the truth in nature.
Contact Information
Akira Nakai, MD&PhD, Professor, Department of Biochemistry and Molecular Biology, Yamaguchi University School of Medicine, Minami-Kogushi 1-1-1, Ube 755-8505, Japan. Phone: 0836-22-2214, Fax: 0836-22-2315, E-mail: anakai@yamaguchi-u.ac.jp